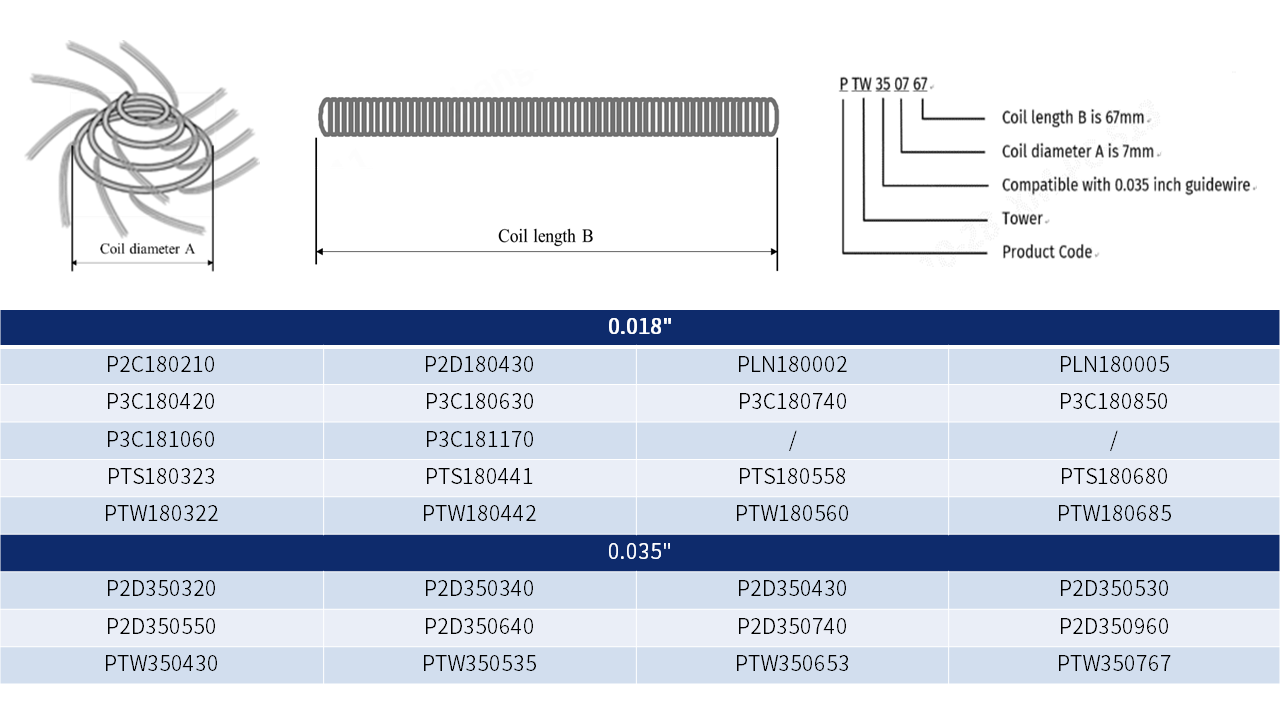
Product Introduction
The HawkNest™ Fibered Embolization Coils are suitable for the treatment of peripheral vascular aneurysms, arteriovenous malformations, and arteriovenous fistulas. It is the first approved embolic product of Endovastec™. The product is available in 0.018" and 0.035" systems and features various configurations including tower (TW), double towers (TS), figure-eight (2C), cloverleaf (3C), multi-loop (2D), and linear (LN). With a wide range of diameters and lengths available, it boasts the most diverse configuration of coils domestically, catering to the clinical needs of various embolization procedures. The coil component is crafted from platinum-tungsten alloy and dense fibers, offering exceptional radiopacity, MRI compatibility, and biocompatibility. A more rational fiber distribution design reduces the risk of stalling . The fibers are processed using a "twisting" technique, facilitating smoother delivery and accelerating thrombosis formation, thereby effectively improving embolization density and reducing the risk of recanalization.
● The product is available in 0.018" and 0.035" systems and features various configurations, with a wide range of diameters and lengths available, catering to the clinical needs of various embolization procedures
● High-density fibers accelerate thrombosis formation, effectively improving embolization density and reducing the risk of re-canalization
● More rational fiber distribution design reduces the risk of stalling during delivery, enhancing both comfort and stability during the procedure
● Composed of platinum-tungsten alloy, offering exceptional radiopacity, MRI compatibility, and biocompatibility
Specification Parameter